Exhaled Nitric Oxide a Potential Biomarker for IPF Severity and Progression, Study Suggests
High levels of nitric oxide in the lungs of people with idiopathic pulmonary fibrosis (IPF) are associated with faster disease progression and worse survival, researchers in Italy report.
The findings were described in the study “Alveolar concentration of nitric oxide as a prognostic biomarker in idiopathic pulmonary fibrosis,” published in the journal Nitric Oxide.
According to the team, the clinical progression of IPF is difficult to predict due to the unavailability of reliable biomarkers.
Studies have suggested that the increased production of nitric oxide in the lungs of IPF patients could cause pulmonary damage, and may represent a reliable and reproducible disease biomarker.
To test this, researchers conducted a retrospective analysis to assess the potential of the alveolar concentration of nitric oxide — levels of nitric oxide in the lung sacs — to predict the likely course of IPF, and serve as a potential prognostic biomarker.
A total of 88 IPF patients (mean age of 65 years), treated at the Siena Regional Referral Centre for Interstitial Lung Diseases between November 2011 and January 2016, were assessed in the study. A group of 60 healthy volunteers (mean age of 63.1 years) were included in the study as controls.
Clinical progression and functional capacity assessments were performed every six months, or according to the patients’ needs.
Pulmonary function was determined by spirometry tests that measure the diffusing capacity of carbon monoxide (DLCO) — the amount of oxygen that passes from the lungs to the blood — and the forced vital capacity (FVC), which is the amount of air that can be forcibly exhaled from the lungs after taking a deep breath.
A DLCO less than 80 – 120% of the predicted value is indicative of poor lung function. A shorter time for FVC to decline to 10% (TTD10) indicates a worse patient’s condition.
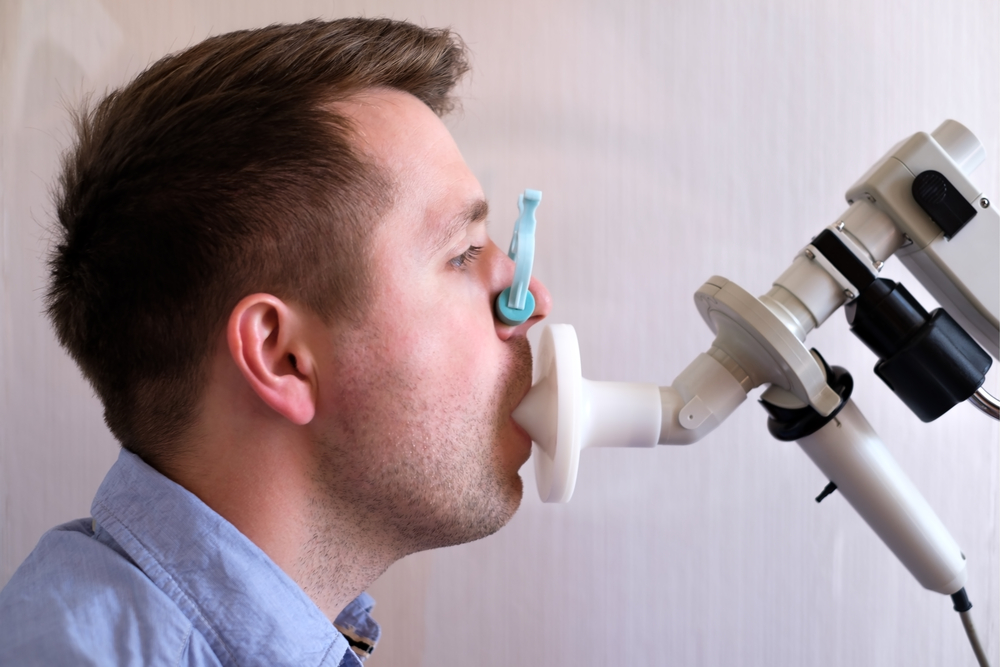
The alveolar concentration of nitric oxide (CaNO) was measured as exhaled nitric oxide using an electrochemical sensor called HypAir FeNO by Medisoft. CaNO levels are reported in parts per billion (ppb) units.
Both the pulmonary function tests and measurement of exhaled nitric oxide are non-invasive procedures, only requiring patients to breathe into the measuring devices.
Results showed that the CaNO levels were significantly higher in IPF patients compared with the healthy control group. Patients’ age, gender, and smoking status did not influence this finding.
Statistical analysis of survival data showed that IPF patients with CaNO levels of six ppb or more had worse outcomes, while those with CaNO levels less than six ppb had an improved survival rate.
The exhaled nitric oxide level also was a strong predictor of IPF progression, as indicated by significantly shorter TTD10 in patients with CaNO levels equal or higher than nine ppb.
“The significantly higher CaNO and its potential prognostic value suggest that NO can be useful not only for insights into the pathogenesis of lung fibrosis but also for predicting disease progression,” the researchers said.
A similar inverse relationship was observed between DLCO and CaNO levels. The team found that there was a statistically significant correlation between DLCO values less than 50% and CaNO greater than 6 ppb. At these values, both parameters predicted poor patient outcome.
The “data suggest that CaNO, a non-invasive and reproducible biomarker, may predict disease progression and survival outcome in patients with IPF,” the team concluded.







