New IPF Therapy Is Goal of Pliant Therapeutics, a Start-up Focusing on Fibrotic Diseases
Redwood City, California-based Third Rock Ventures, LLC, a healthcare venture firm, recently
announced $45 million in Series A financing to launch the biotechnology start-up company Pliant Therapeutics, Inc., with a mandate to discover, develop, and commercialize breakthrough drug treatments for fibrotic diseases.
Pliant’s product engine has the potential to address the needs of many patients by targeting fibrosis in a variety of organs and conditions, including the lungs (idiopathic pulmonary fibrosis, IPF), liver (NASH and cirrhosis), kidneys (renal fibrosis), skin (scleroderma), heart (cardiac fibrosis), and the gastrointestinal tract (Crohn’s disease), according to a press release.
Pliant’s initial primary focus is on IPF, a rare but lethal disease that currently affects approximately 200,000 people in the U.S., and results in some 40,000 deaths annually. Patients diagnosed with IPF experience progressive difficulties in breathing and, eventually, complete respiratory failure. Currently available avenues of treatment are scarce, and have no proven effect on long-term patient survival or symptomatology.

Fibrosis results from the accumulation of scar-like tissue in vital organs, causing irreparable damage. Pliant Therapeutics’ primary objective is the development of small molecule therapeutics to attack key mechanisms underlying the fibrotic process through employing the as-yet largely untapped therapeutic potential of integrin biology to modulate and prevent activation of transforming growth factor beta (TGF-ß). The company believes TGF-ß is arguably the most critical regulator of physiological healing and pathologic fibrosis, and of epithelial-to-mesenchymal transition — the main driver of activated fibroblasts generated from normal epithelial cells.
Pliant’s lead program aims to develop a small molecule inhibitor for IPF that modulates the v1 integrin on fibroblasts, blocking the activation of TGF-ß in a tissue-specific manner, thereby preventing the growth of fibrotic tissue within the lung. Pliant has entered into a license agreement with the University of California, San Francisco (UCSF), to expand this technology, and expects to initiate company-sponsored IND-enabling studies for this program in 2017.
TGF-ß is a family of cytokines, multifunctional peptides that regulate proliferation, differentiation, adhesion, migration, and other functions in cells. Many cells have TGF-ß receptors, and the protein positively and negatively regulates many other growth factors.
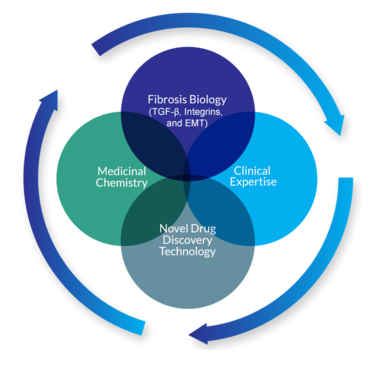 Pliant’s approach will focus on the antagonism between cell- and tissue-specific TGF-ß signaling, which, in turn, is anticipated to arrest and possibly reverse the fibrotic effects of TGF-ß. Pliant researchers note that while targeting TGF-ß broadly has produced unwanted systemic side effects, the researchers hope that by targeting TGF-ß signaling in a tissue-specific manner through integrin inhibition, they will be able to develop means of effectively modulating the fibrotic cascade, thereby maximizing therapeutic effects without inciting adverse events. Additionally, Pliant is building a pipeline of cell-specific small molecule inhibitors of the pathological epithelial-to-mesenchymal-transition (EMT) process that is induced by TGF-ß.
Pliant’s approach will focus on the antagonism between cell- and tissue-specific TGF-ß signaling, which, in turn, is anticipated to arrest and possibly reverse the fibrotic effects of TGF-ß. Pliant researchers note that while targeting TGF-ß broadly has produced unwanted systemic side effects, the researchers hope that by targeting TGF-ß signaling in a tissue-specific manner through integrin inhibition, they will be able to develop means of effectively modulating the fibrotic cascade, thereby maximizing therapeutic effects without inciting adverse events. Additionally, Pliant is building a pipeline of cell-specific small molecule inhibitors of the pathological epithelial-to-mesenchymal-transition (EMT) process that is induced by TGF-ß.
Pliant aims to target many fibrotic diseases through novel mechanisms, with IPF as its first indication. It is hoped that the dual approach of tissue-specific TGF-antagonism and EMT inhibition will provide a novel and potentially synergistic therapeutic option to treat fibrotic disorders.
Pliant has additional programs in IPF leveraging small molecule inhibitors of integrins on epithelial cells as a second tissue-specific approach to modulating TGF-ß activation. Epithelial cells have emerged as principal players in IPF pathophysiology, and the researchers say their animal models confirm that selectively targeting TGF-ß activation in these cells holds therapeutic promise.
Patient Registry & Biomarker Discovery
In addition to translating scientific advances in the biology behind fibrosis into therapies for patients in need, the new company is committed to building a multicenter patient registry that collects clinical and laboratory data on individuals with IPF and other fibrotic diseases, to create a resource that will help increase understanding of natural disease progression and stimulate biomarker discovery. Through this approach, Pliant intends to design efficient clinical trials leveraging validated clinical endpoints.
Management Team
Dr. Bernard Coulie, who has been appointed Pliant’s chief executive officer, brings more than 15 years of drug development expertise, including six years in leadership positions at Johnson & Johnson, and  most recently as co-founder and CEO of ActoGeniX. Dr. Coulie holds an MD and PhD from the University of Leuven in Belgium, is a board-certified internist, and received his MBA from the Vlerick Management School, also at Leuven.
most recently as co-founder and CEO of ActoGeniX. Dr. Coulie holds an MD and PhD from the University of Leuven in Belgium, is a board-certified internist, and received his MBA from the Vlerick Management School, also at Leuven.
 Perry Karsen, who has more than 30 years of experience in the biopharmaceutical industry, has been selected as chairman of the Pliant board of directors after his recent retirement as CEO of Celgene Cellular Therapeutics, serving alongside Third Rock Ventures partner Neil Exter and venture partner Charles Homcy, MD.
Perry Karsen, who has more than 30 years of experience in the biopharmaceutical industry, has been selected as chairman of the Pliant board of directors after his recent retirement as CEO of Celgene Cellular Therapeutics, serving alongside Third Rock Ventures partner Neil Exter and venture partner Charles Homcy, MD.
Pliant’s management team also includes David Morgan s, PhD, vice president of drug discovery and early development, who brings more than 25 years of experience in drug discovery and development of small molecules.
s, PhD, vice president of drug discovery and early development, who brings more than 25 years of experience in drug discovery and development of small molecules.
Craig Muir, interim chief technology officer and partner at Third Rock Ventures, who also has more than 25 years of experience in technology development, implementation, and application under his belt in the biotechnology and pharmaceutical industries, is also part of Pliant’s management team; along with Richard Gaster, MD, PhD, physician, scientist and entrepreneur, who joined Pliant as director of translational medicine from Third Rock Ventures, where he served as a senior associate responsible for new company formation.
more than 25 years of experience in technology development, implementation, and application under his belt in the biotechnology and pharmaceutical industries, is also part of Pliant’s management team; along with Richard Gaster, MD, PhD, physician, scientist and entrepreneur, who joined Pliant as director of translational medicine from Third Rock Ventures, where he served as a senior associate responsible for new company formation.
Scientific Team

 Scientific members of the Pliant team include Dean Sheppard, MD, a UCSF professor of medicine and chief of the Division of Pulmonary, Critical Care, Allergy and Sleep; Bill DeGrado, PhD, a UCSF professor in the Department of Pharmaceutical Chemistry; Hal Chapman, MD, a professor of medicine, Division of Pulmonary, Critical Care, Allergy and Sleep at UCSF; and Bradley Backes, PhD, a UCSF associate professor in the Department of Medicine.
Scientific members of the Pliant team include Dean Sheppard, MD, a UCSF professor of medicine and chief of the Division of Pulmonary, Critical Care, Allergy and Sleep; Bill DeGrado, PhD, a UCSF professor in the Department of Pharmaceutical Chemistry; Hal Chapman, MD, a professor of medicine, Division of Pulmonary, Critical Care, Allergy and Sleep at UCSF; and Bradley Backes, PhD, a UCSF associate professor in the Department of Medicine.

 The UCSF Pliant Technology licensing deal was negotiated by UCSF’s Office of Innovation, Technology & Alliances (ITA), which coordinates UCSFs forging of collaborations and licensing of technologies that can translate science breakthroughs in the lab on campus into therapies and products that can fill unmet needs of patients worldwide.
The UCSF Pliant Technology licensing deal was negotiated by UCSF’s Office of Innovation, Technology & Alliances (ITA), which coordinates UCSFs forging of collaborations and licensing of technologies that can translate science breakthroughs in the lab on campus into therapies and products that can fill unmet needs of patients worldwide.
“Pliant’s founders include world-renowned research ers from the University of California, San Francisco who have discovered key insights into the biology behind fibrosis and developed small molecule therapeutics to target this devastating disease process,” said Third Rock Ventures partner Neil Exter. “Third Rock Ventures is very excited to have Dr. Coulie join these esteemed experts to bring forward an entirely new approach for fibrosis patients who currently have limited options.”
ers from the University of California, San Francisco who have discovered key insights into the biology behind fibrosis and developed small molecule therapeutics to target this devastating disease process,” said Third Rock Ventures partner Neil Exter. “Third Rock Ventures is very excited to have Dr. Coulie join these esteemed experts to bring forward an entirely new approach for fibrosis patients who currently have limited options.”
Sources:
Third Rock Ventures
Pliant Therapeutics, Inc.
University of California, San Francisco (UCSF)






