Idiopathic Pulmonary Fibrosis Found To Cause Evolutionarily Ancient “Master” Protein That Protects Injured Cells, Repairs Damage
Written by |
Researchers at Brown University in Providence, Rhode Island and collaborating institutions report having achieved some progress in the battle against idiopathic pulmonary fibrosis (IPF), a devastating lung disease that afflicts millions of people worldwide. Two experimental drugs have recently showed promise in clinical trials, and now a study last week in the American Association for the Advancement of Science journal Science Translational Medicine offers both an unprecedentedly thorough explanation of how IPF progresses, and introduces another potential therapeutic avenue.
The new study is focused on an evolutionarily ancient protein called “chitinase 3-like-1” (CHI3L1) which the study coauthors identify as the “master regulator” of what appears to be a tragically errant repair response to the mysterious lung injuries that give rise to the disease. In describing how CHI3L1 works in IPF, the research also points to a strategy for treatment.
The study paper, entitled “Chitinase 3Like 1 Suppresses Injury and Promotes Fibroproliferative Responses in Mammalian Lung Fibrosis” (Sci Transl Med 11 June 2014: Vol. 6, Issue 240, p. 240ra76 DOI: 10.1126/scitranslmed.3007096) is coauthored by Yang Zhou, Huanxing Sun, Xueyan Peng, Chuyan Tang, Ye Xiaosong, Chen Gan, Aditi Mathur, Buqu Hu, Martin D. Slade, Chang-Min Le, Meagan W. Moore, Mridu Gulati, Chun Geun Lee, and of the Section of Pulmonary, Critical Care and Sleep Medicine, Department of Medicine, Yale School of Medicine, New Haven, CT; Hong Peng of the Department of Respiratory Medicine, The Second Xiangya Hospital of Central South University, Changsha, China; Ruth R. Montgomery and Albert C. Shaw of the Program on Aging, Yale School of Medicine; Robert J. Homer of the Department of Pathology, Yale School of Medicine; Eric S. White of the Department of Medicine, University of Michigan School of Medicine, Ann Arbor, Michigan; and Corresponding author (with Erica L. Herzog ) Jack A. Elias of the Division of Biology and Medicine, Warren Alpert School of Medicine at Brown University, Providence, RI
The coauthors observe that epithelial injury, alternative macrophage accumulation, and fibroproliferation coexist in the lungs of patients with idiopathic pulmonary fibrosis (IPF), and that Chitinase 3like 1 (CHI3L1) is a prototypic chitinase-like protein that has been retained over species and evolutionary time. However, they note that regulation of CHI3L1 in IPF, and its ability to regulate injury and/or fibroproliferative repair have not been fully defined.
[adrotate group=”3″]
In their study, the researchers report that they were able to demonstrate that CHI3L1 levels are elevated in patients with IPF, noting that high levels of CHI3L1 are associated with progression — as defined by lung transplantation or death — and with scavenger receptorexpressing circulating monocytes in an ambulatory IPF population.
They report that in preterminal acute exacerbations of IPF, CHI3L1 levels were reduced and associated with increased levels of apoptosis, and that they also demonstrated that in bleomycin-treated mice, CHI3L1 expression was acutely and transiently decreased during the injury phase and returned toward and eventually exceeded baseline levels during the fibrotic phase.
Encouragingly, in this model the scientists showed that CHI3L1 played a protective role in injury by ameliorating inflammation and cell death, and a profibrotic role in the repair phase by augmenting alternative macrophage activation, fibroblast proliferation, and matrix deposition. Using three-dimensional culture system of a human fibroblast cell line, the researchers found that CHI3L1 is sufficient to induce low grade myofibroblast transformation, concluding that in combination, these studies demonstrate that CHI3L1 is stimulated in IPF, where it represents an attempt to diminish injury and induce repair, and also that high levels of CHI3L1 are associated with disease progression in ambulatory patients and that a failure of the CHI3L1 antiapoptotic response might contribute to preterminal disease exacerbations.
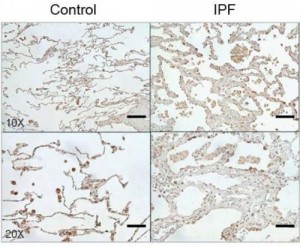
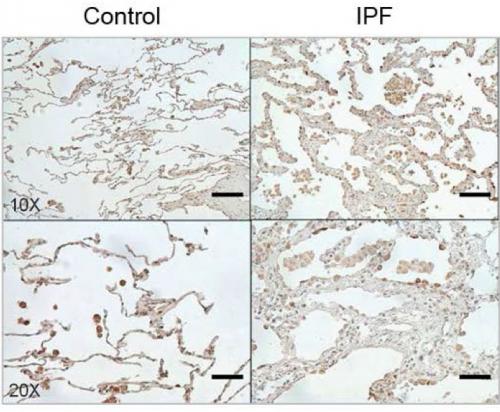
Image Caption: The protein CHI3L1 works to protect injured cells and to repair damage. In lung tissue, damage repair means a buildup of scar tissue, which compromises the lung. Levels of CHI3L1 are higher in patients with idiopathic pulmonary fibrosis (right) than in healthy controls. Credit: Brown University/Yale University
The report demonstrates that CHI3L1 is produced to help in response to the injury. It feeds back to protect injured cells from dying and simultaneously stimulates tissue repair to patch the damage that has occurred. But the study also shows how this dual role contributes to the ultimate problem. If IPF resulted from a single injury, like a paper cut, CHI3L1 would decrease the injury and cause local scarring while it restored tissue integrity. In that case, the amount of scarring would not be excessive and tissue function would not be significantly altered. But in IPF lungs, cells undergo ongoing injury, so CHI3L1 is chronically elevated and scar tissue accumulates. As CHI3L1 rescues cell after cell, the scarring builds up, eventually compromising the lung’s ability to breathe. In IPF, 70 percent of patients die within five years.
“The CHI3L1 is doing exactly what it is supposed to do it is designed to shut off cell death and decrease injury,” says Dr. Jack A. Elias, a co-senior author of the study and dean of medicine and biological sciences at Brown University. Dr. Elias is joined on the paper by a host of his former colleagues and students at Yale University where the research occurred. “But at the same time it is decreasing cell death it is driving the fibrosis. You’ve got this ongoing injury so you’ve got these ongoing attempts to shut off injury which stimulate scarring.”
The research team, including co-senior author Erica Herzog of Yale and co-lead authors Yang Zhou (who is transitioning to Brown from Yale), Huanxing Sun of Yale, and Hong Peng of Central South University in China used various means to uncover CHI3L1’s central role in IPF.
 Dr. Herzog’s laboratory at Yale focuses on the relationship between chronic inflammation,
Dr. Herzog’s laboratory at Yale focuses on the relationship between chronic inflammation,  neuronal guidance proteins, and lung fibrosis in a variety of fibrosing lung diseases including IPF, Scleroderma and sarcoidosis. Her unique translational approach to these diseases combines transgenic murine modeling and bioengineering with studies of primary human cells.
neuronal guidance proteins, and lung fibrosis in a variety of fibrosing lung diseases including IPF, Scleroderma and sarcoidosis. Her unique translational approach to these diseases combines transgenic murine modeling and bioengineering with studies of primary human cells.
The researchers compared tissues and serum from normal patients, outpatients with IPF, and patients with an acute exacerbation (AE) of IPF. In AE, widespread lung injury is superimposed on the pulmonary fibrosis, which frequently occurs before patients die. In lung biopsies and serum, they found that CHI3L1 levels are elevated in both tissue compartments in the outpatients with IPF and that the levels of CHI3L1 correlated with their disease progression. In the patients with AE, elevated levels of CHI3L1 were not noted, showing that the levels of CHI3L1 decrease right before the patients die. “This demonstrates that the CHI3L1 plays a key role in controlling lung injury in this setting,” Dr. Ellias notes in a Brown release..
After documenting that elevated levels of CHI3L1 correlate with ongoing fibrosis and scarring and that a lack of the protein associates with widespread cell death, the team engaged in several manipulations of CHI3L1 in mice to see how levels and the clinical outcomes might be related. (In mice, CHI3L1 is also called BRP-39.)
Scientists can induce an IPF-like response using a drug called bleomycin in mice, in which the researchers found levels of CHI3L1 declined at first, then surged. When the protein levels were low, cell damage occurred, and when the protein surged, excessive scarring set in. In previous research the team had engineered several lines of genetically modified mice. Some were transgenic and can produce CHI3l1 on chemically delivered command, while others were engineered to never produce BRP-39 — the mouse version of CHI3L1 — at all.
Using these mice, the researchers found if they triggered CHI3L1 production early after administering bleomycin, the mice fared well, experiencing less injury, less damage and less scarring than controls. However, if they waited several days after bleomycin to trigger CHI3L1, the mice fared very poorly and scarring and mortality increased.
Mice who couldn’t produce CHI3L1/BRP-39, had acute lung cell damage, analogous to AE patients who have a relative deficiency of CHI3L1. However, without CHI3L1 they did not generate much scarring.
These findings were supplemented with several other experiments designed to demonstrate how CHI3L1 interacts with other cells involved in tissue repair response in both human and mouse lungs. The experiments, including studies conducted in a bioengineered 3-D model of lung tissue seeded with relevant cells, showed that CHI3L1 regulates a pathway that recruits cells, such as macrophages and fibroblasts, that produce the scarring, or fibrosis. In all, the results show that CHI3L1 plays a fundamental role in the course, if not the origin, of IPF. An ongoing buildup of it results in excessive scarring. Too little and cells die much more frequently.
“To my knowledge this is the first comprehensive paper that’s been able to explain the many facets and presentations of IPF,” Dr. Elias observes. “It explains and links the injury and the repair responses that are critical in the disease. It also provides an explanation for the slowly progressing patients and the patients that experience acute exacerbations.”
Toward Treatment
Dr. Elias says he hopes the insights learned from the research will lead to new IPF therapies whose objective would be to preserve the cell-protecting function of CHI3L1, while tempering its ability to stimulate tissue scarring and repair.
“There may indeed be a way to do that,” Dr. Elias explains. “Some data suggest that the mechanisms for each CHI3L1 function cell protection and tissue repair involve different pathways and or receptors. In people, therefore, separate drugs could hypothetically enhance the injury prevention pathway and temper the repair pathway. Indeed, drugs that block a key repair pathway receptor exist and are undergoing testing in other diseases. This research lays the foundation for potential therapies that would be designed to diminish injury and ameliorate fibroproliferative repair.”
The National Institutes of Health (HL109033, UO1-HL-108638, 5U01HL112702 and R01-HL-093017), The American Thoracic Society, the Pulmonary Fibrosis Foundation, and Yale University supported the research. Dr. Elias has submitted a patent related to CHI3L1.
Sources:
Brown University
Science Translational Medicine
Yale University
Image Credits
Brown University
Yale University