Phase 1 Clinical Trial May Suggest Positive Clues On Drug Candidate For IPF
Written by |
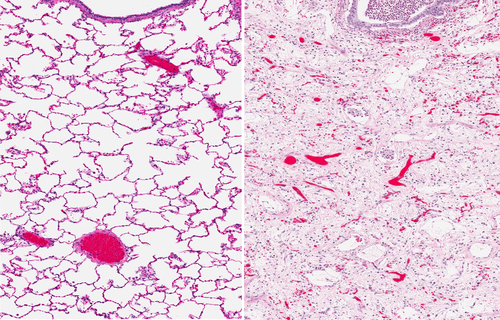
 Pacific Therapeutics Ltd., a pharmaceutical company focused on the reformulation of existing approved drugs, recently announced the end of an initial phase 1 trial of its drug candidate for Idiopathic Pulmonary Fibrosis, PTL-202, combined with an important antioxidant amino acid, with positive signs on its effectiveness, low side effects, and small dose need.
Pacific Therapeutics Ltd., a pharmaceutical company focused on the reformulation of existing approved drugs, recently announced the end of an initial phase 1 trial of its drug candidate for Idiopathic Pulmonary Fibrosis, PTL-202, combined with an important antioxidant amino acid, with positive signs on its effectiveness, low side effects, and small dose need.
According to the information released by Accesswire, previously, the results of experiments in a standard animal model of pulmonary fibrosis induced by bleomycin seemed to suggest that PTL-202 is safe and effective agent for the treatment of pulmonary fibrosis, a disease that may be on the rise.
The phase 1 clinical trial of PTL-202 intended to test the interaction between the Active Ingredients combined in PTL-202 with synergistic effects and to assess new side effects.
The results showed an interactive relationship resulting in an increase in both the Active Ingredients of PTL-202 in the blood and in known therapeutic effects without any new side effects, which may mean the possibility for patients to take smaller doses improving the chance of marketing approval.
The tests showed that the amount of Active Ingredients in the blood of healthy males was much higher if given in combination than if they were given alone. At the same time, the already known therapeutic effects like vasodilation were also higher with an increased amount of Active Ingredient in the blood.
[adrotate group=”3″]
These synergetic interactions may also represent the possibility of getting a once-a-day smaller dose in the final product, resulting in a more “patient friendly” pill, smaller and easier to swallow.
The existing approved forms of the Active Ingredients are required 2 or 3 times a day and may release the ingredients to0 fast, which may eventually represent high levels of the drugs in the blood at one time and possibly increasing side effects.
According to the press release, the positive phase 1 results combined with positive efficacy results from the pre-clinical testing of PTL-202 in animals with pulmonary fibrosis may call for a second clinical trial of PTL-202.
The company may turn its focus to determine the exact amount of Active Ingredients in the final formulations, because if the ratio of Active Ingredients is not precise or the Active Ingredients are released into the body too quickly, the drug’s effectiveness may be reduced or the side effects increased.
IPF is a serious and potentially fatal disease that affects more than 100,000 Americans and for which there is no cure. According to the National Institutes of Health (NIH), the disease affects the lungs, making them thick, stiff, and scarred, forming fibrosis and seriously complicating the breathing process and the oxygen body transportation. The disease develops fast or slow, according to each patient, but many of them end up dying after 3 to 5 years after they are diagnosed, especially from respiratory failure.






