Promedior’s PRM-151 for Myelofibrosis Passes into Stage 2 of Phase 2 Trial
Written by |
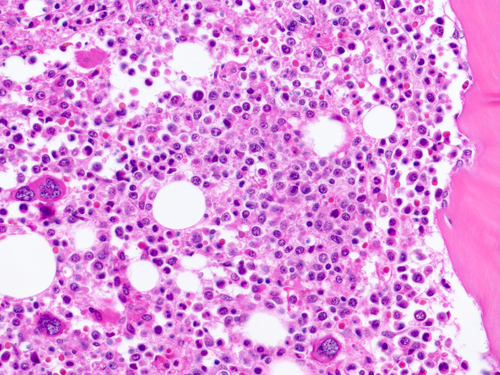
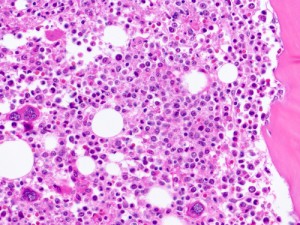 Promedior, Inc. presented at the American Society of Hematology (ASH) meeting positive results from Stage 1 of its adaptive two-stage Phase 2 trial investigating PRM-151 use in patients with myelofibrosis. The treatment, a novel anti-fibrotic immunotherapeutic, yielded an overall response rate of 43% after 6 months of administration, which greatly surpassed the pre-specified efficacy criteria needed to begin Stage 2.
Promedior, Inc. presented at the American Society of Hematology (ASH) meeting positive results from Stage 1 of its adaptive two-stage Phase 2 trial investigating PRM-151 use in patients with myelofibrosis. The treatment, a novel anti-fibrotic immunotherapeutic, yielded an overall response rate of 43% after 6 months of administration, which greatly surpassed the pre-specified efficacy criteria needed to begin Stage 2.
“These promising clinical data in myelofibrosis in patients highlight the differentiated benefits of PRM-151, showing an unprecedented rate of reversing bone marrow fibrosis,” said Suzanne L. Bruhn, PhD, President and Chief Executive Officer of Promedior, in a company news release. “Further, by validating PRM-151’s novel mechanism of action to reverse fibrosis, this study demonstrates the broad potential in a range of other fibrotic diseases.”
Other results identified a reduction of bone marrow fibrosis by at least one grade in 42% of treated patients, correlating to improvements in anemia and thrombocytopenia. “We are encouraged by the favorable safety profile and clinical activity of PRM-151 demonstrated in the first stage of this trial, and are excited to see that a reduction of bone marrow fibrosis by PRM-151 is associated with signs of improved hematopoiesis,” said Srdan Verstovsek, MD, PhD, principal investigator and ASH presenter. “There is tremendous enthusiasm for PRM-151 among investigators and patients, and we look forward to moving forward with Stage 2 of this important clinical trial.”
[adrotate group=”3″]
Eleven of 25 patients able to be evaluated in Stage 1 already showed other improvements that included 4 IWG-MRT Clinical Improvement Symptom responses and reduced splenomegaly. Twenty-seven patients with primary myelofibrosis, post-polycythemia vera MF, or post-essential thrombocythemia have already enrolled in the trial, and more are being sought for Stage 2 of the trial.
So what’s next in line for Promedior? “We will continue to move PRM-151 forward as a new treatment option for patients with myelofibrosis and other fibrotic diseases,” said Dr. Bruhn. “We expect to initiate the next stage of PRM-151’s Phase 2 clinical program in myelofibrosis in the first half of 2015.”



