Small Peptide Shows Promise in Reducing Lung Fibrosis in a Mouse Model
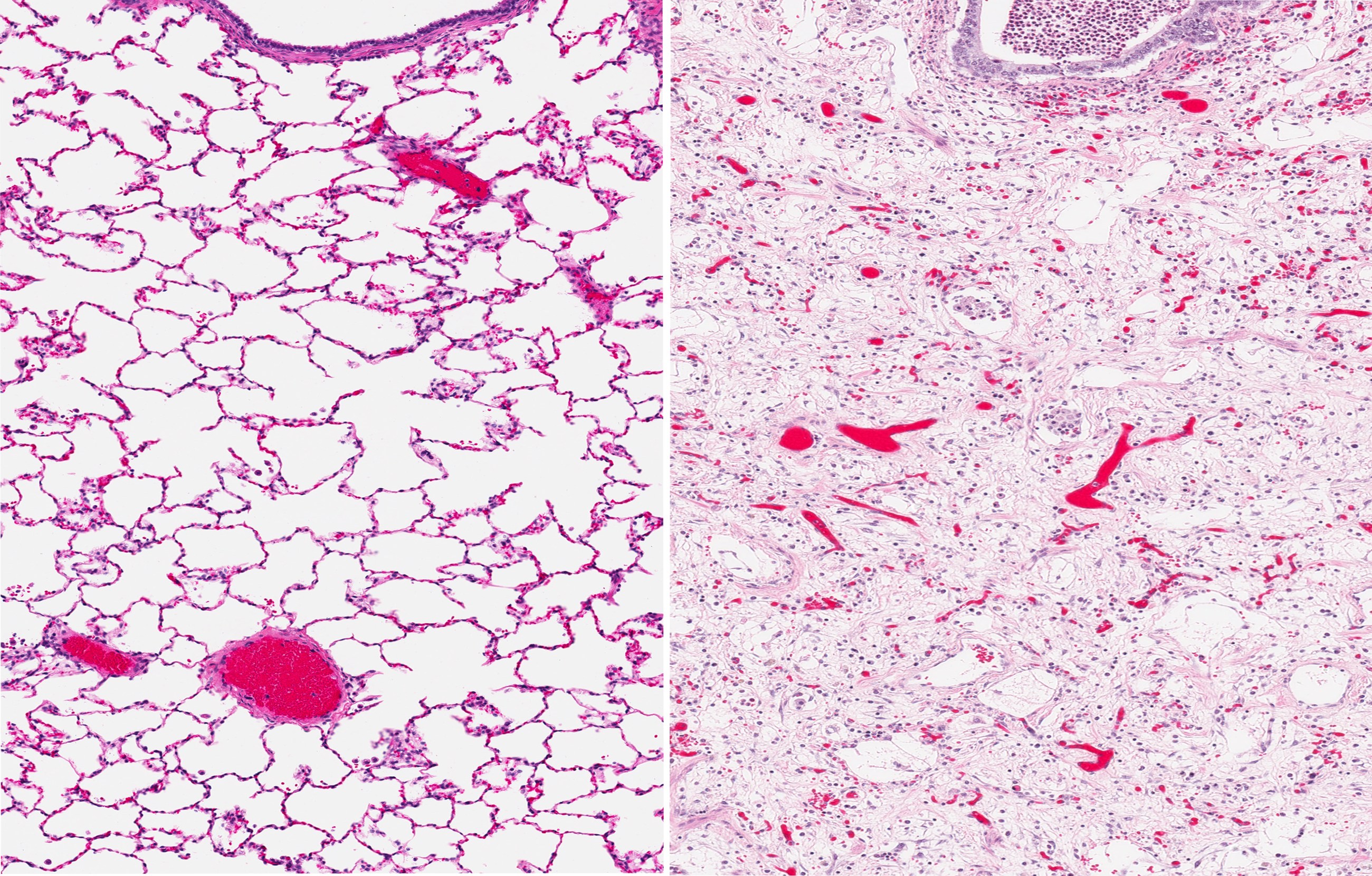
Researchers at the Medical University of South Carolina found that M10, a naturally occurring small peptide, appeared to effectively reduce lung fibrosis in a mouse model of systemic sclerosis, a finding that might lead the way to new treatments for lung fibrosis.
Systemic sclerosis can be viewed as the archetypal fibrotic disease, often serving as a model for other fibrotic illnesses. In the study, “M10, a caspase cleavage product of the hepatocyte growth factor receptor, interacts with Smad2 and demonstrates antifibrotic properties in vitro and in vivo,” a research team induced lung fibrosis in mice using the drug bleomycin. Findings, published in the journal Translational Research, indicated that M10 impacted the signaling of TGF-β, a main fibrosis-promoting signaling molecule.
“Systemic sclerosis is often more than skin deep, affecting the gastrointestinal tract, the lungs, the heart, the kidneys, and the blood vessels, so it is a model for many other more prevalent fibrotic diseases,” said Richard M. Silver, director of the Division of Rheumatology and Immunology at MUSC and a co-author on the article, in a press release. “Whereas there may be 300,000 Americans with scleroderma/systemic sclerosis, millions of others suffer from fibrosis of these other organ systems.”
Studies have previously suggested that M10 has anti-fibrotic properties, but the mechanisms behind its effects have eluded scientists. Researchers treated the fibrotic mice with M10, and observed that it slowed fibrosis development.
To evaluate if the peptide could alter collagen production by fibroblasts — cells highly contributing to disease — they isolated skin and lung fibroblasts from three people with systemic sclerosis, all deceased, and cultured the cells in the lab. These fibroblasts produced more collagen than healthy ones, but when treated with M10, the production was reduced. It was also clear that the higher the dose of the M10 peptide used, the more the collagen production was reduced.
The team repeated the experiment in fibroblasts from healthy individuals, and treated the cells with TGF-β to trigger collagen production. Again, M10 was seen to reduce the release of collagen.
Findings suggested that the M10 peptide directly interacts with the TGF-β signaling pathway, likely by interacting with proteins downstream of TGF-β, and blocks its actions.
“We observed that treatment with M10 by intraperitoneal injection markedly improved bleomycin-induced lung fibrosis in mice, suggesting that the M10 peptide may have potential for use in the treatment of scleroderma-associated interstitial lung disease and other forms of pulmonary fibrosis, for example, idiopathic pulmonary fibrosis,” said Galina S. Bogatkevich, senior study author.
In the experiments, M10 was given at the same time as bleomycin, so their outcome demonstrates the preventive effect of the peptide. The team is now planning to evaluate if M10 can also be used when fibrosis is already established.
If M10 turns out to have anti-fibrotic properties in future experiments, Dr. Bogatkevich and her team hope to find a partner within the pharmaceutical industry to develop a drug for clinical use.







