[vc_row][vc_column][vc_column_text]PRM-151 is a recombinant human serum amyloid P/pentraxin 2 protein developed by Promedior, a biotechnology company developing therapies for fibrotic diseases using plasma-derived proteins and peptides. Also referred to as PTX2, the therapy injected intravenously in patients, is showing promise as an effective anti-fibrotic agent that can be used to target several rare, orphan diseases with unmet therapeutic needs.
Promedior is currently focusing on rare systemic fibrotic diseases. The company’s prime targets include idiopathic pulmonary fibrosis (IPF), myelofibrosis and retinal fibrovascular diseases.
History of PRM-151
PRM-151 is granted Orphan Drug Designation by the United States Food and Drug Administration (FDA) and the European Commission (EC). Following the designation, Promedior conducted Phase 1 clinical trials on healthy volunteers to test the drug for safety and tolerability, and in patients with IPF to test safety, efficacy and tolerability of PRM-151 in mild to moderate cases. It was observed in the studies that PRM 151 significantly helped in increasing the Forced Expiratory Volume (FEV1) and lung capacity of patients at the end of eight weeks of the trial, after being administered on a dose-dependent basis for two weeks. The results were presented at the American Thoracic Society Annual Meeting on May 22, 2013, which paved the way for enrollment and execution of a Phase 2. The Phase 2 study is in the planning stages.
How PRM-151 Works
Pentraxin-2 is an endogenous human protein that plays an important role in regulating the response to fibrosis. It directs the immune system to naturally turn off and reverse the process of fibrosis, which occurs as a result of excess collagen secretion and cellular growth and differentiation. Unlike other formulations that work by stopping a single target on the downstream side of fibrosis, this protein works by reversing and possibly healing the fibrotic tissue.
Research suggests that it may localize specifically to sites of injury and function to aid in the removal of damaged tissue. Combined with the ability to regulate monocyte differentiation (another inflammatory regulator), it has been proven to have ‘first-class’ effects in reversal of fibrosis and promotion of healing.
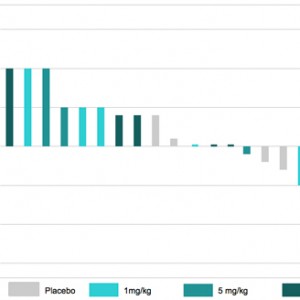
PRM-151 in Patients with IPF:
Relative change from Baseline on Day 57 in FVC % Predicted
[/vc_column_text][/vc_column][/vc_row][vc_row][vc_column width=”1/2″][vc_wp_text title=”Sign Up For Our IPF Clinical Trial Registry:”]
Pulmonary Fibrosis News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
s