[vc_row][vc_column][vc_column_text]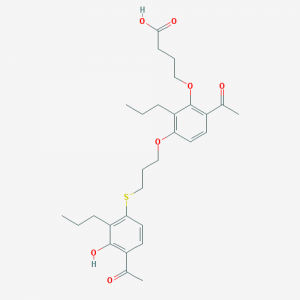 Tipelukast (MN-001) is an oral macromolecular formulation developed originally by Kyorin Pharmaceuticals and now in clinical trials in the United States by MediciNova.
Tipelukast (MN-001) is an oral macromolecular formulation developed originally by Kyorin Pharmaceuticals and now in clinical trials in the United States by MediciNova.
Licensing rights were acquired in 2002.
MediciNova was recently granted an official license to commercially manufacture and sell the drug worldwide except in Japan, China, Taiwan and South Korea. The drug is under patent protection until 2023.
Called MN-001 in the pharmaceutical industry, tipelukast is primarily an anti-fibrotic and anti-inflammatory drug formula that was initially designed to treat interstitial cystitis and asthma. It is currently fast-tracked by the U.S. Food and Drug Administration (FDA) for clinical trials as a treatment for fibrotic liver diseases that include non-alcoholic steatohepatitis (NASH), pulmonary fibrosis, and fatty liver disease among others.
History of Tipelukast
The drug was tested successfully in 2006 in patients with mild to moderate asthma, and then moved into Phase 3 clinical trials to test different doses of the drug in patients with moderate to severe asthma. The trials so far have proven tipelukast’s efficacy and tolerance in patients without major side-effects, prompting MediciNova to continue testing the drug for inflammatory diseases.
How Tipelukast Works
Modes of action for tipelukast include leukotriene (LT) receptor antagonism, inhibition of phosphodiesterases (PDE) (mainly 3 and 4), and inhibition of 5-lipoxygenase (5-LO); down-regulation of genes that promote fibrosis, such as LOXL2, collagen type 1 and TIMP-1; and genes responsible for promoting inflammation like CCR2 and MCP-1. Preclinical investigations involving the administration of tipelukast on a dose-dependent basis in animal models demonstrated abilities at the pre-clinical level.
Next Steps For Tipelukast
MediciNova is moving forward with a Phase 2 clinical trial on humans with fibrotic and inflammatory liver conditions. The company applied to the FDA for an Investigational New Drug (IND) Application to the Division of Pulmonary, Allergy, and Rheumatology Products (DPARP) in January 2015. It was granted fast-track status for testing in humans in April.
Note: Pulmonary Fibrosis News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
[/vc_column_text][/vc_column][/vc_row][vc_row][vc_column width=”1/2″][vc_wp_text title=”Sign Up For Our IPF Clinical Trial Registry:”]




