Researchers Uncover Groundbreaking Insights Into Fibrosis
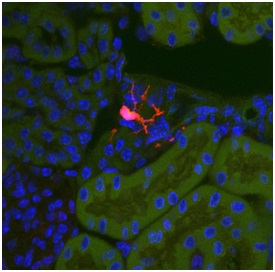
A Gli1 progenitor cell (red) located in a healthy kidney. During fibrosis these cells differentiate into myofibroblasts, causing scarring and organ failure. Photo credit: Rafael Kramann, MD
 In a new study entitled “Perivascular Gli1+ Progenitors Are Key Contributors to Injury-Induced Organ Fibrosis” a team of researchers identified a new key factor for the formation of fibrotic tissue. The study was published in the journal Cell Stem Cell. The findings could eventually lead to new therapies that address fibrotic conditions found in diseases such as pulmonary fibrosis.
In a new study entitled “Perivascular Gli1+ Progenitors Are Key Contributors to Injury-Induced Organ Fibrosis” a team of researchers identified a new key factor for the formation of fibrotic tissue. The study was published in the journal Cell Stem Cell. The findings could eventually lead to new therapies that address fibrotic conditions found in diseases such as pulmonary fibrosis.
Fibrosis is a process by which the body produces an excess of connective tissue to be deposited in an organ or tissue. While this can be performed as a response to an injury, it can often block the normal functioning of the organ or tissue in question, such as with the kidneys, lungs, liver and heart. When an injury occurs, tissue-resident fibroblasts are activated and are transformed into myofibroblasts – they secrete extracellular matrix components and were suggested to be key players to the formation of fibrotic tissue.
In this study, the authors studied a specific type of cell – mesenchymal stem cells (MSCs) – which reside in organs and often register the occurrence of fibrosis, particularly in the heart and lungs, but whose functions in vivo remain largely unclear. The mesenchymal stem cells are a group of cells that possess stem cell characteristics — specifically, self-renewal and clonogenic capacity and reside closely to blood vessels. As fibrosis was previously suggested to result from the accumulation and activation of myofibroblasts, the authors tracked the cell origin of these myofibroblasts. They identified that tissue-resident MSC-like cells specifically expressed a gene – Gli1 – belonging to the hedgehog pathway. The authors observed that upon kidney, lung, liver, or heart injury these tissue-resident MSC-like Gli1 positive cells increased their proliferation by approximately 20-fold and transformed into myofibroblasts, suggesting their role in fibrosis.
[adrotate group=”3″]
By genetically ablating these MSC-like Gli1 positive cells from tissues, the authors observed a reduced formation of fibrotic tissue in mice models of kidney and heart fibrosis, therefore establishing the role of these cells to injury-induced organ fibrosis.
Now, the team of researchers at the Researchers from Brigham and Women’s Hospital (BWH) aim to develop drugs that can selectively target and shut-down the fibrotic-induced cells, with the potential to find drug selective candidates for clinical use in humans. These findings would impact drug development for IPF in particular, as these new findings are closely related to the fibrosis experienced in the lungs — the hallmark of the disease.
Benjamin Humphreys, MD, PhD, a physician scientist in the Renal Division at BWH and study leading author commented, ““We’ve found that these Gli1 progenitor cells differentiate into myofibroblasts, and in fibrotic disease, when they are ablated, we can rescue organs and organ function. Most organs develop fibrosis as we age. Specifically, in the kidney we lose one percent of kidney function as a result of fibrosis for each year that we age. We look forward to future research, using human tissue, to confirm our findings in humans and work to develop a potential therapy.”







