Home Oxygen Therapy No Longer Requires In-Person Testing and Signature Due to COVID-19 Risk
The Centers for Medicare and Medicaid Services (CMS) has waived all requirements for in-person testing and signature at delivery for supplemental oxygen during the COVID-19 pandemic.
The Pulmonary Fibrosis Foundation (PFF) is urging patients, healthcare providers, and oxygen suppliers to comply with the new CMS guidelines, in order to protect patients with lung diseases, such as pulmonary fibrosis (PF), while ensuring that they receive the care and supplies they need at home.
“These CMS rule adjustments will allow patients to remain as safe as possible and avoid exposure to the virus that causes COVID-19, while continuing to receive lifesaving oxygen,” William T. Schmidt, PFF president and CEO, said in a press release. “We were proud to lead several national healthcare organizations in advocating for public policies that will improve the lives of our patients and caregivers.”
PF causes progressive scarring of the lungs, a process that causes lung tissue to become stiff, affecting lung function, and leading to deficient oxygen transport into the bloodstream. Low levels of oxygen are responsible for characteristic symptoms of the disease, including shortness of breath, persistent dry cough, and fatigue.
Oxygen therapy is prescribed to many PF patients, as it helps increase blood oxygen levels and improves breathing.
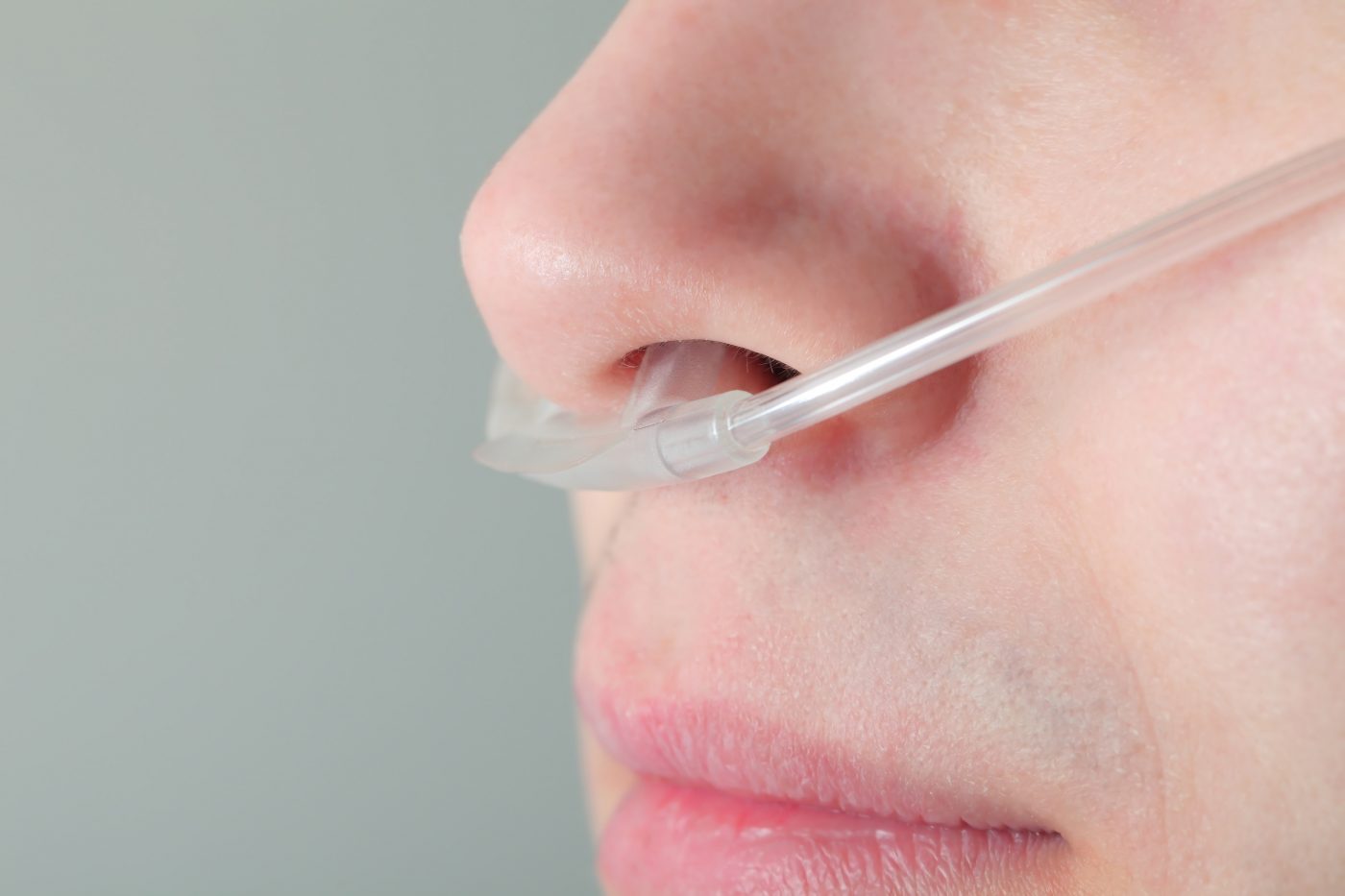
Oxygen is typically administered by a thin tube that is placed in the nostrils, and attached to a tank filled with pure oxygen. Commonly, patients use a large tank or cylinder at home.
To initiate therapy or re-certify prescriptions, the CMS requires in-person testing and signature at delivery of supplemental oxygen.
CMS received an urgent request to change the supplemental oxygen therapy protocol to reduce the risk of potential exposure to COVID-19 for patients. The joint letter was sent by PFF, together with the American Association for Respiratory Care, American Lung Association, American Thoracic Society, COPD Foundation, LAM Foundation, National Association for the Medical Direction of Respiratory Care, Pulmonary Hypertension Association, and Three Lakes Foundation.
The new guidelines, issued March 30, will be effective during the COVID-19 public health emergency, and will allow more flexibility to healthcare staff to care for their patients.
The new indications include items such as NCD 240.2 Home Oxygen, LCD L338000 respiratory assist devices, NCD 240.5 Intrapulmonary Percussive Ventilator, and LCD L33797 Oxygen and Oxygen equipment. The complete CMS policy on respiratory services can be found on the COVID-19 Public Health Emergency Statement, pages 128-129.
CMS has also expanded its telehealth services for Medicare beneficiaries, allowing healthcare facilities to help more patients remotely.






