AlloSure Lung tests, Medicare billing, and the tale of David and Goliath
How advocacy can lead to positive results for patient and medical communities

How much of a powerhouse in the world of rare disease medicine do you have to be to effect change?
Advocacy is tough, especially when you’re trying to influence organizations that are responsible for federal policy. So where to start?
How about starting with a single voice?
The biblical story of David and Goliath has been widely embraced as a classic tale of good versus evil. In a modern setting, the story could be applied to someone who takes on a large corporation, government bureaucracy, or a nonsensical rule or regulation.
Malcolm Gladwell wrote “David and Goliath: Underdogs, Misfits, and the Art of Battling Giants” in 2013 to illustrate the power of the underdog. While I don’t know how many copies have sold, I do believe the book has inspired a certain degree of bravado in people from all walks of life.
When I was diagnosed with idiopathic pulmonary fibrosis (IPF) in early 2017, it was the greatest challenge I had ever faced. Little did I know that by stepping up to face that challenge, I would confront many of the same issues that people in the rare disease community navigate every day.
At the time, I was already familiar with rare diseases, such as ataxia, a progressive neuromuscular disorder that affected my stepsiblings — three of whom passed away from it. Because of this, I had been involved with the ataxia community for many years. I served on the board of the National Ataxia Foundation, including as board president. I understood rare diseases.
To those in the IPF community, the disease is our Goliath. To fight those kinds of battles, we need the proper tools.
Fighting for what is best for us
In May, I wrote about AlloSure Lung, a test that assists in identifying potential indications of organ rejection in lung transplant patients. Early detection of organ rejection allows a care team to address the issues sooner. As that column was being published, I learned from a survey administered by three organizations aligned with the transplant community that Medicare had indicated in a billing instruction that tests like AlloSure Lung would not be covered under the program.
If you have never read Medicare billing instructions before, they can be confusing. For that reason, a letter from the American Society of Transplant Surgeons in response to the billing instruction helped explain why tests like AlloSure are so important to the medical and patient communities.
Honor the Gift — the coalition of organizations I mentioned above — also advocated for Medicare to reverse its position on the AlloSure test. To convince Medicare decision-makers that the test is essential preventive medicine, it was necessary to rally community support.
The survey that caught my attention was hosted by Honor the Gift members Transplant Recipients International Organization, Transplant Families, and the HeartBrothers Foundation. It noted that of nearly 1,200 responders — who were patients, caregivers, and family members — 95% said they were concerned that post-transplant care would be negatively affected if Medicare didn’t cover the test. More than 90% felt it would be a financial burden. You can view the full survey at Honor the Gift.
Personally, it was confounding to me that Medicare, which covered the cost of my bilateral lung transplant, would consider eliminating a proven test that can provide early detection of organ rejection. Goliath, meet David!
Winning the battle
In July, a breakthrough happened. A press release by CareDx, the company that produces the AlloSure Lung test, announced that Medicare had capitulated and agreed to cover it.
“We are 100% committed to transplant patients and today’s milestone approval represents a monumental day for lung transplant patients,” CareDx CEO Reg Seeto said. “Notably, in 2021, we were the first in the field to deliver this type of non-invasive solution to monitor rejection in lung transplantation. We did so because of the significant unmet need where it is estimated that 1 in 2 lung transplant patients are expected to have their transplant fail five years post-transplant.”
By changing its position, Medicare indicated an understanding of the importance of this test to both the medical and patient communities. Your voice made a difference.
Standing up for what is important to the lung transplant community is necessary to protect the gift we have received. I have always believed the AlloSure Lung test is an important part of my care plan. The information provided by AlloSure Lung to my care team (see table below) is necessary to allow them to identify signs of rejection early. Standing up with other patients for what is right and necessary is how I make every breath count.
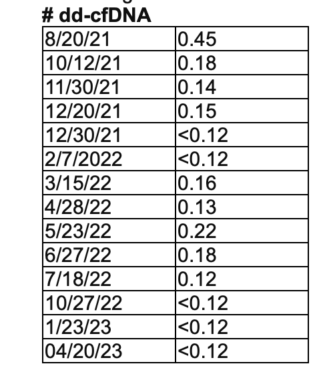
According to Sam’s care team, his AlloSure Lung test levels are excellent. (Courtesy of Sam Kirton)
Note: Pulmonary Fibrosis News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. The opinions expressed in this column are not those of Pulmonary Fibrosis News or its parent company, Bionews, and are intended to spark discussion about issues pertaining to pulmonary fibrosis.








Paul Lowe
I can relate to this article. I went through this Battle with MED
icare Advantage Plan in early 2023 to no avail. I will ask the doctor to try again.
Samuel Kirton
Hi Paul,
Thanks for reading my column and your comment. All insurance has variables and I hope you are able;e to resolve your situation.
Sam ...