Pulmonary Fibrosis News Forums › Forums › Treatments and Science › Medscape – The Future of IPF
-
Medscape – The Future of IPF
Posted by Larry70 on March 19, 2024 at 7:44 pmA great podcast (that is transcribed if you rather read) on future developments in the treatment of IPF. https://www.medscape.com/viewarticle/982426?fbclid=IwAR1NK9imq6VDEq6zJtQuiELLiWzybXlUc7fUbhqJdyypwarp3gNafVOWYac
medscape.com
Episode 6: Now Unfolding: The Future of Idiopathic Pulmonary Fibrosis
In this podcast, Drs Jeffrey Swigris and Toby Maher discuss the future of idiopathic pulmonary fibrosis. Find out where we're headed regarding diagnostic and treatment innovations on the near horizon.
anonymous replied 1 year ago 7 Members · 7 Replies -
7 Replies
-
This is an informative discussion regarding research surrounding medical trials for IPF patients. It provides patients with IPF with an honest insight from a doctors point of view of life expectancy and the hope that there will be new treatments to stop the developement of IPF not just slow the progression.
-
I read on this website and through other media about many new drugs for IPF. Most of the are in phase 2-3. They have had very good results with a lot of them. But , with the FDA, they will not be available for 5 years. By that time most of us suffering from this disease will be dead!!! Why can’t the more serious cases of IPF have these new drugs now as a last resort!!!
-
I do agree – it would at least give hope where is very little at present. The Covid drugs are a good example – they were devised and circulated and taken up by multi millions of people – surely with AI this can be considered earlier?
-
Great information, however, I agree with the other replies…too long to wait. Why can’t we opt to try some of these treatment options in the later trials.
-
The drug company for one of the drugs mentioned in the podcast recently announced they will start the phase 3 trial as soon as they have their max participants enrolled in phase 2b in their attempt to help speed things up. Don’t give up hope. There’s also a possibility of compassionate use for drugs that haven’t gotten to market. Keeping the faith!!
-
(a condensed version of what ChatGPT 4o gave me) ‘There is a lengthy review process after Phase 3 completion that can take several months or longer. For a drug to become available – it typically ranges from 1 to 2 years, depending on a number of regulatory considerations by the FDA and other review processes. For drugs that qualify for expedited pathways like fast track, breakthrough therapy, priority review, or accelerated approval, this timeline can be shortened. This pathway to market is crucial to ensuring that new drugs are both safe and effective when they reach the public. Each step is designed to rigorously test these aspects under regulatory oversight, minimizing potential risks to patients.’
My hope is that my Ofev will keep the progression at a very slow pace and that the phase 3 drugs that have recently been completed may come to market before the disease has advanced too far.
-
The Fibroneer Phase 3 trial ends in November. Its an FDA breakthrough drug (speedier process). According to this “lengthy review process after Phase 3 completion that can take several months or longer” so it should be available early next Spring IF the results show it works as well as it did in Phase 2 (where it worked very well). This is ONE CASE but it does look like this drug MIGHT work well… we shall see in less than a year!
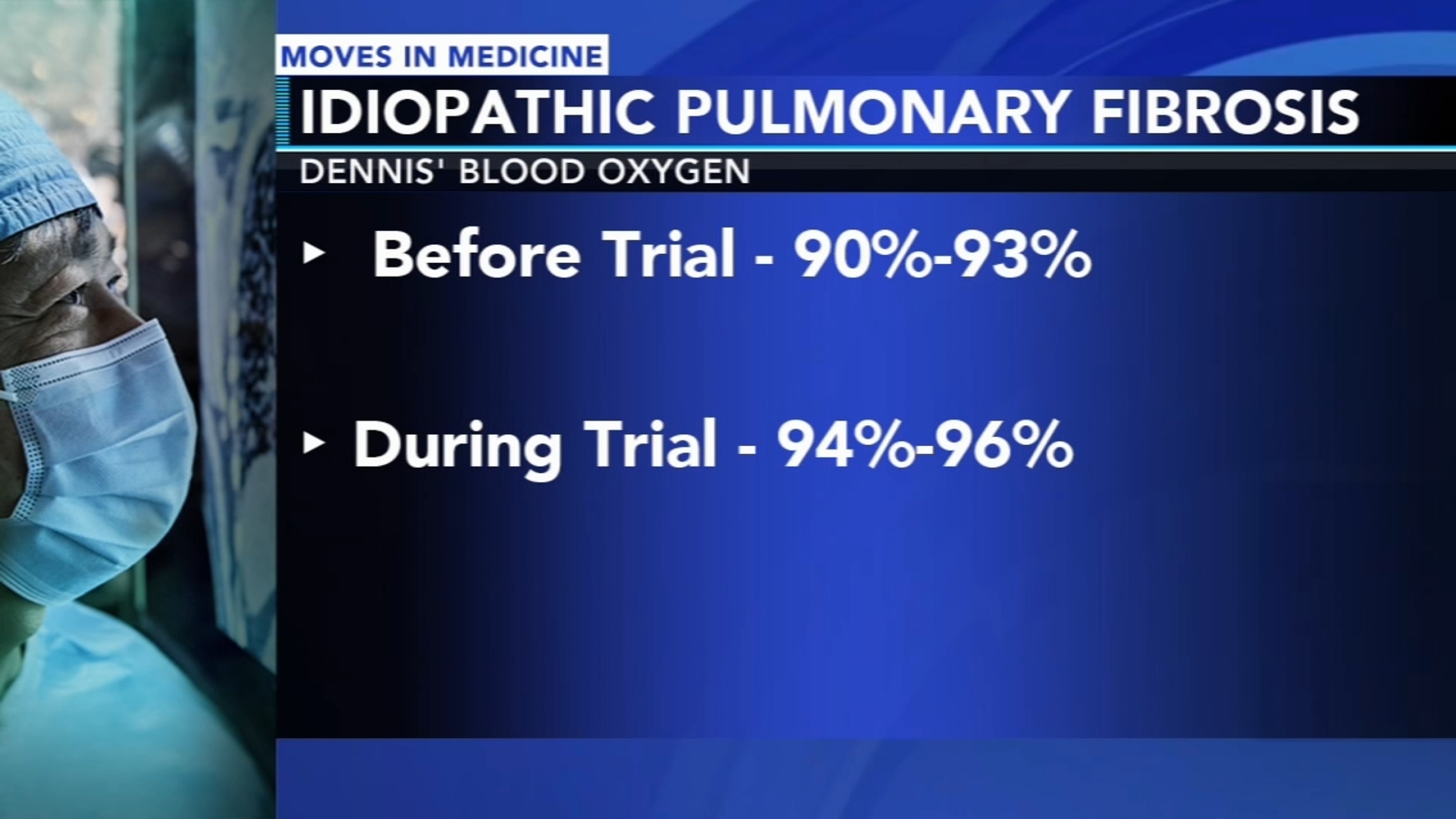
https://6abc.com/temple-lung-center-idiopathic-pulmonary-fibrosis-scar-tissue-breathe/13577878/
6abc.com
Fibroneer could aid in treating pulmonary fibrosis, stop lung scarring
Pulmonary fibrosis turns the lungs to scar tissue, making it harder and harder to breathe. Help may be on the way as a drug being tested locally aims at giving sufferers their breath back.
Log in to reply.